Under FDA regulations, individuals with a life-threatening illness or serious conditions with no other available treatment options can request access to investigational therapies through the Expanded Access (EA) pathway. WCG IRB provides prompt review and oversight for single-patient EA requests, as a pro bono service.
During the COVID-19 pandemic (since the start of 2020), WCG IRB has received almost twice the number of single-patient EA requests compared to the prior two years. All EA requests reviewed by WCG IRB were approved. WCG IRB’s experience with EA submissions over the past 4 years is summarized here, with consideration of the impact of the COVID-19 pandemic.
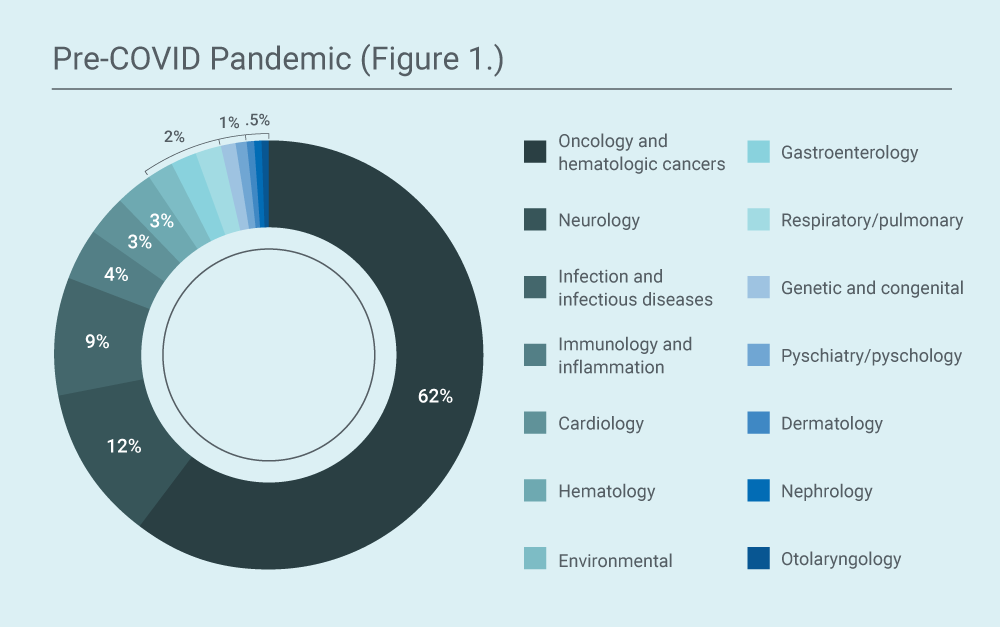
Pre-COVID-19 pandemic (2018-2019):
During this period, most of the requests WCG IRB received were for oncology and oncology-related indications, which accounted for 62% of the total requests. The next most frequent therapeutic area for which single-patient EA was requested was neurology, which accounted for 12% of the total requests, followed by infections and infectious diseases with 9% of the total requests. Many of these requests were for clofazimine for the treatment of mycobacterial infections (leprosy); while clofazimine is FDA-approved for this indication, the incidence of the infection is so low in the United States that it is only available directly from the manufacturer through expanded access rather than by prescription.
All other therapeutic areas contributed less than 3% of the total number of requests. While EA pathways are often thought of as being only for people who are critically ill, the submissions received also included treatments for long-term conditions such as gastroparesis, eczema, and chronic mercury toxicity.
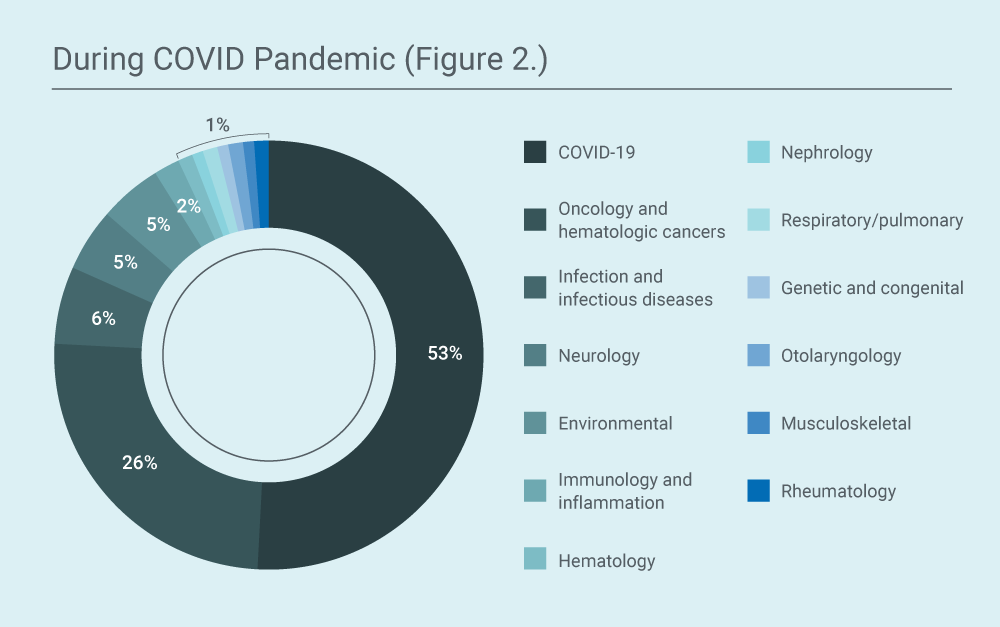
During COVID-19 pandemic (2020 vs 2021):
Requests for COVID-19 therapies or preventative agents accounted for 53% of the total number of requests in 2020-2021 (64% in 2020 and 30% in 2021). The COVID-19 agents for which EA was requested changed over time, as investigational agents became available through either large-scale group EA programs (which are conducted like clinical trials) or through FDA Emergency Use Authorization (EUA) or FDA approval. For example, remdesivir accounted for 55% of the COVID-19-related requests and convalescent plasma for 27% of the COVID-19- related requests in 2020, but there were no requests for either product in 2021, after they had become available through other routes. In 2021, most requests (55%) were for the monoclonal antibody cocktail casirivimab and imdevimab. The other requests were for antibody-based antiviral agents, stem cells, antibiotics, synthetic peptides, and for access to COVID-19 vaccines (after EUA, but for people who were not yet eligible according to public health guidelines).
During 2020-2021, 26% of the total requests (59% of the non-COVID-19 requests) were for oncology or oncology-related indications. The next most frequent therapeutic area in which WCG IRB received EA requests was in infections and infectious diseases, which accounted for 6% of the total requests (13% of the non-COVID-19 requests), followed by the environmental therapeutic area (mercury toxicity, for the provision of an investigational chelating agent) which accounted for 4% of the total requests (9% of the non-COVID-19 requests). The next most frequent therapeutic area for which single-patient EA was requested was neurology which accounted for 4% of the total requests (9% of the non-COVID-19 requests).
Though the requests for EA for the treatment of oncology and oncology-related indications seems to have decreased during COVID-19 pandemic (62% vs 26%), there was no significant decrease in percentage when compared to the total number of non-COVID-19 requests (62% pre-COVID-19 vs 59% during COVID-19) and the absolute number of requests year-to-year were consistent. This pattern was similar for other therapeutic areas such as neurology and infections and infectious diseases.
Don't trust your study to just anyone.
WCG's IRB experts are standing by to handle your study with the utmost urgency and care. Contact us today to find out the WCG difference!

