The Most Experience + The Largest Network = A Winning Combination
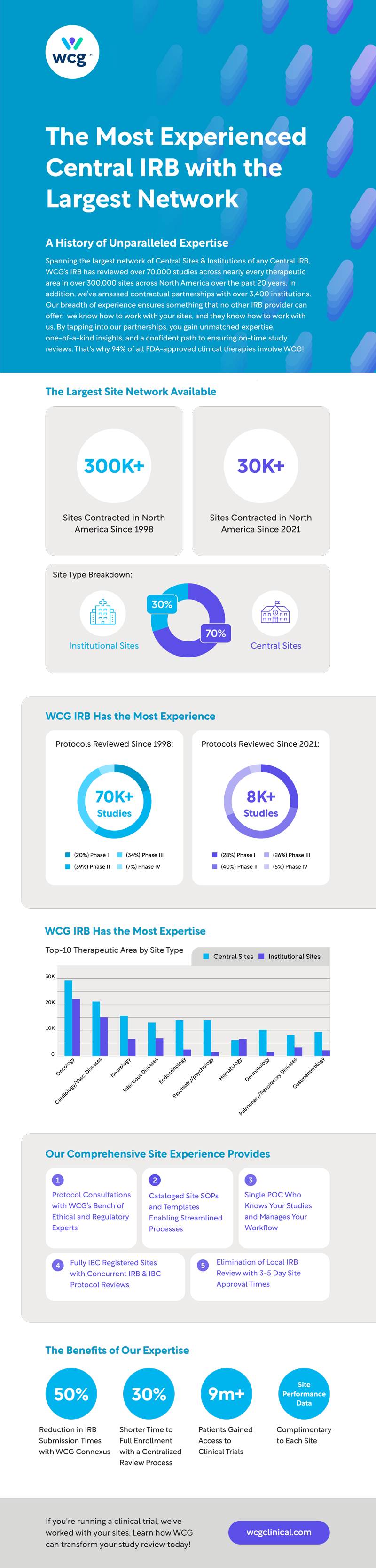
A History of Unparalleled Expertise
Spanning the largest network of Central Sites & Institutions of any Central IRB, WCG’s IRB has reviewed over 70,000 studies across nearly every therapeutic area in over 300,000 sites across North America over the past 20 years. In addition, we’ve amassed contractual partnerships with over 3,400 institutions.
Our breadth of experience ensures something that no other IRB provider can offer: we know how to work with your sites, and they know how to work with us. By tapping into our partnerships, you gain unmatched expertise, one-of-a-kind insights, and a confident path to ensuring on-time study reviews. That’s why 94% of all FDA-approved clinical therapies involve WCG!
The Largest Site Network Available:
Sites contracted in NA since 1998
Sites contracted in NA since 2021
Central Sites
Institutional Sites
WCG’s IRB Has the Most Experience
Protocols reviewed since 1998:
70,000+ Studies
- (20%) Phase I
- (39%) Phase II
- (34%) Phase III
- (7%) Phase IV
Protocols reviewed since 2021:
8,000+ Studies
- (28%) Phase I
- (40%) Phase II
- (26%) Phase III
- (5%) Phase IV
Our Comprehensive Site Experience Provides:
- Protocol consultations with WCG’s bench of ethical and regulatory experts
- Cataloged site sops and templates enabling streamlined processes
- Single POC who knows your studies and manages your workflow
- Fully IBC registered sites with concurrent IRB & IBC protocol reviews
- Elimination of local IRB review with 3-5 day site approval times
The Benefits of Our Expertise:
reduction in IRB submission times with WCG Connexus
shorter time to full enrollment with a centralized review process
patients gained access to clinical trials
Site performance data complimentary to each site
If you’re running a clinical trial, we’ve worked with your sites. Learn how WCG can transform your study review today!
Don't trust your study to just anyone.
WCG's IRB experts are standing by to handle your study with the utmost urgency and care. Contact us today to find out the WCG difference!