Today’s clinical research landscape is growing, but pressures on clinical trial sites are growing faster. While the COVID-19 pandemic resulted in many clinical trials being halted during 2020, there was still a 15.4% increase in new trial initiations between Q4 2019 and Q4 2021. When comparing figures year over year, that increase was even larger at 17.6%. Oncology trials had the biggest growth rate at about 23% year over year.1
Site Staff Shortages
But while demand for clinical trial startups is increasing, the available staff to manage them is decreasing. Early in the pandemic, in April and May 2020, when many elective procedures and clinical trials were put on hold, involuntary staff reductions (through layoffs, furloughs, and early retirements) occurred, amounting to about 12% of the workforce at the hospital or institutional site level. Then as trial initiations started to ramp up in 2021, another staff exodus occurred. Dubbed “the great resignation,” these were voluntary actions, and almost one in five healthcare workers left their job. That 18% voluntary staff reduction came on top of the 12% reduction that occurred the year before, compounding the staff shortage. Then in late summer 2021, when COVID-19 vaccine mandates were implemented, that impacted another 1-2% of the hospital workforce and exacerbated the “site crunch.”2
By the end of January 2022, news headlines reported that about 11% of US hospitals had reached critically low staffing levels, impacting not just their research departments, but also their ability to provide patient care. An additional 21% of hospitals stated that they were teetering on the brink of critically low staffing levels. A recent Becker’s headline reported that staffing was hospital CEOs’ number one concern for the first time in 17 years.3
The staffing issues that have occurred at hospitals and institutions are a cause for concern. WCG has also been using them as a proxy to understand what is happening in research departments. That insight has been supplemented with WCG surveys conducted throughout 2020 and 2021. In early 2021, sites stated that trial enrollment was their number one concern and staffing was second. Towards the end of 2021, staffing became the primary concern at about 75% of the sites. That is not surprising as 19% of sites reported a staff turnover of 30% or higher.
The magnitude of this staff reduction represents an immense challenge, resulting in many sites having to put existing trials on hold or not being able to reopen trials they put on hold in 2020. Furthermore, there is a bolus of new studies coming into the pipeline and trials that cannot be started.
This is not only a US phenomenon. They are also seeing staffing shortages in Europe, but not nearly as severe as the current US levels. Outside the US, about 44% of sites state that staffing is a concern. That is the figure for research professionals, but physicians are also critically important stakeholders in these programs. In the US, 97% of physicians do not enroll in clinical trials. That is a major issue that the industry must try to resolve.
Between 2017 and 2020, there was a 34% reduction in the number of investigators that were participating in clinical trials. In 2021, the number of investigators involved improved, perhaps due to increased awareness of the need for clinical advances during the pandemic, but the ratio of physicians to new trial starts has not gained much momentum. Furthermore, 41% of physicians participate in only one clinical trial.1
Expediting Site Startups
Sponsors require highly qualified sites to conduct their clinical trials and site startup speed is incredibly important. As a result, 90% of all clinical trials are conducted at one of the top 50 institutions. WCG Institutional Review Board (IRB) is used 75% of the time for those institutions’ clinical trials, regardless of whether it was chosen as the central IRB.
WCG IRB has a very close relationship with its sites, which allows it to better understand some of their site-specific behaviors. Furthermore, WCG does not only manage the sites’ IRBs, but it also provides many other services that help to accelerate trial startups.
Currently, WCG has Master Service Agreements with more than 3,300 US institutions – hospitals, academic centers, and universities – and about 99% of independent or community practice sites. This is the largest and most well-established site relationship network of any central IRB provider. It has well defined onboarding processes at all those sites and systems for managing individual industry-sponsored studies. It often has teams embedded at sites that can align processes to improve speed too.
Real-World Example
Consider a real-world example in which a top 5 pharmaceutical sponsor is beginning a Phase 2 oncology study at 30 US research sites. Figure 1 shows the time and cost savings the sponsor could have enjoyed if it had deployed WCG IRB to reduce turnaround times (TATs). But the sponsor chose a competitor IRB, which did not have a comparable institutional bandwidth. That decision resulted in avoidable delays in the study startup timeline.
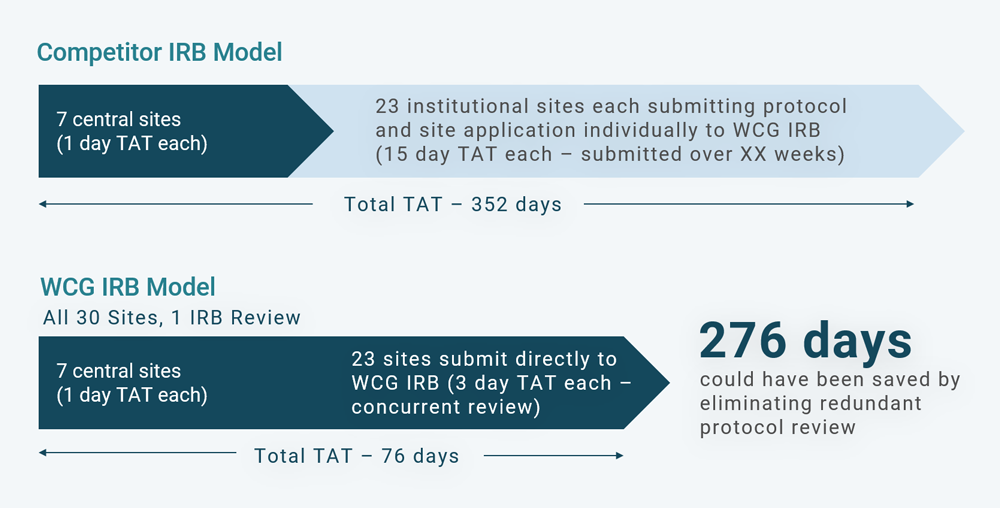
In this example, there were 30 sites, seven of which were private practice sites that any central IRB could review and approve. Those central sites usually have a protocol review TAT of about one business day each. Then the sponsor selected 23 institutional or local IRB sites. Those 23 institutional sites deferred their study reviews to WCG. But because WCG wasn’t selected as the central IRB for that study, all 23 sites had to go through an independent full board IRB review at WCG, which take about 15 days each for a total of 345 days. With the addition of the seven one-day reviews for the central sites, that made a total of 352 days in startup time.
If WCG had been chosen as the central IRB, all 30 sites would have been included under WCG’s umbrella review of the protocol, informed consent, and other study documentation. There would have been an eight-day study review, but each site could then have been gone through WCG’s expedited review and would not have needed to go to a fully convened board. The seven central sites would still have been reviewed in about one day each, but the 23 sites would have taken three days each instead of 15 days each, resulting in a startup TAT of 76 days instead of 352 days, a saving of 276 days.
Some people are not aware that academic centers and hospitals are deferring their IRB reviews to WCG, so it is a good question to ask prior to study initiation.
WCG IRB’s Site Relationships
Using WCG IRB’s site relationships introduces many efficiencies into the site startup process. As WCG already knows the sites and their trial preferences, it can quickly review the protocol and refer it to the sites that would have interest in it for a feasibility assessment. Once that is complete, WCG moves the process forward with one contract, one budget, and one payee for all the sites. That step removes a significant burden for the sites. It is also a lot easier for the sponsor or clinical research organization because it now has a single contract with WCG instead of one with all the individual sites. WCG has conducted more than 2,000 studies using this model and has had more than 1,000 clinical trial agreements executed.
WCG does a lot of upfront work, profiling sites, learning about their staff, level of physician engagement, and therapeutic areas of interest. It also reviews sites’ past success rates in terms of participant enrollment. This site knowledge expedites the contracting and budgeting processes.
If a study requires as institutional biosafety committee (IBC) review in addition to an IRB one, that is conducted through WCG IBC and there is still just one contract, one budget, and one payee.
This WCG support is particularly important now because the “site crunch” does not refer only to clinical research coordinators, but also to regulatory team members, and those working on contracts and budgets. When fully implemented, this streamlined, expedited approach can reduce the site study startup process, up to enrolling the first patient, by about 30%.
Conclusion
WCG is helping clinical trials to get up and running efficiently by augmenting site staff, facilitating centralized IRB reviews and IBC reviews, and implementing a network model that has successfully reduced enrollment timelines. This multi-faceted approach is enabling safe and effective new therapies to get to patients faster.
References:
- WCG Knowledge Base, 2022
- Becker’s Hospital Review, 10/4/21
- Becker’s Hospital Review, 2/4/22